Scientific Article by Lecturer Haider Mutlaq Musa Title: Gas Chromatograph (GC) and Its Applications in Chemical and Biological Analysis
Introduction
The Gas Chromatograph (GC) is a fundamental analytical instrument in chemical and biological laboratories. It enables the separation of chemical components in a mixture and the identification of each compound individually, providing precise information about the chemical composition of samples. GC operates by passing a carrier gas through a column containing a stationary phase, where compounds move at different rates depending on their physical and chemical properties.
Principle of Operation
GC separation is based on differences in retention time for each compound within the column. The mixture is injected into the column, carried by a carrier gas (such as helium or nitrogen) through a stationary phase. Compounds interact differently with the stationary phase, eluting at distinct times. Their signals are detected by a detector, generating a chromatogram that indicates the identity and quantity of each compound.
Main Components of GC
Injection System (Injector)
Converts the sample into vapor before entering the column.
Can be Split or Splitless, depending on the sample volume.
Column
Can be a Capillary Column or a Packed Column.
Packed with stationary phase to separate compounds according to their chemical and physical interactions.
Carrier Gas
Common gases: helium, nitrogen, or hydrogen.
Responsible for transporting compounds through the column.
Detector
Examples: FID (Flame Ionization Detector), TCD (Thermal Conductivity Detector).
Converts eluted compounds into electrical signals, recorded by a computer.
Data Acquisition and Processing System
Software or display screen to visualize the chromatogram and calculate compound concentrations.
Types of Measurements in GC
Quantitative Analysis: Determining the concentration of each compound using calibration curves.
Qualitative Analysis: Identifying compounds based on retention times and comparison with standards.
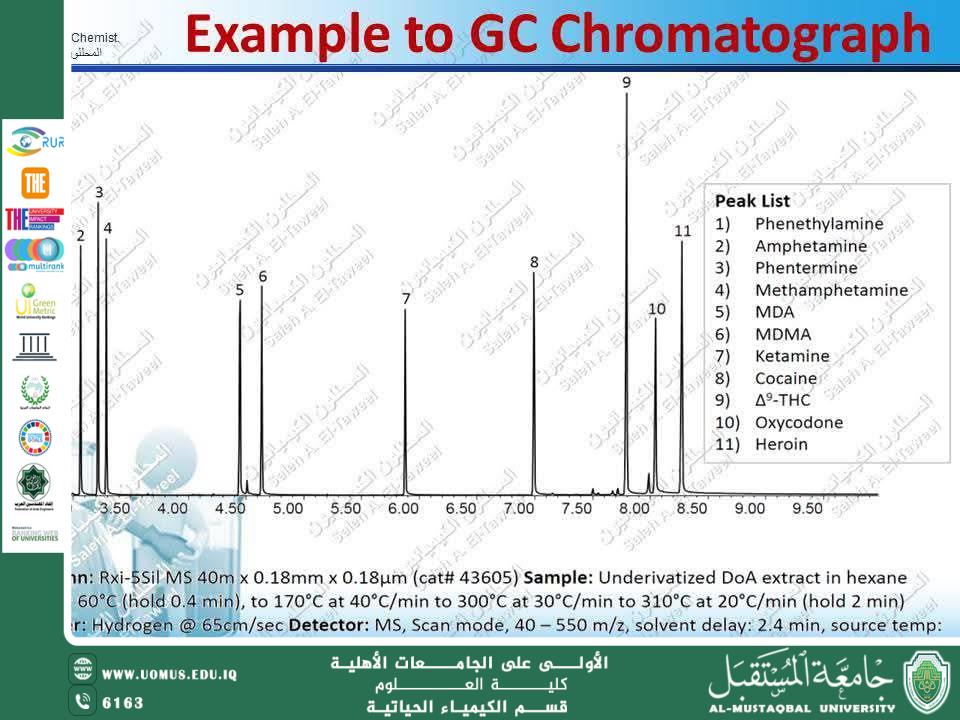
Complex Sample Analysis: Combining GC with mass spectrometry (GC–MS) to obtain detailed structural information.
Applications in Biochemistry
Analysis of fatty acids in foods and oils.
Measurement of hormones and vitamins in biological samples.
Monitoring environmental pollutants such as pesticides and organic solvents.
Separation of complex organic compounds in medical and pharmaceutical research.
Practical Examples of GC Use
Measuring volatile oils in plants using FID.
Determining alcohols and sugars in food samples.
Detecting toxic compounds in drinking water and industrial waste.
Advantages
High-resolution separation of different compounds.
Ability to analyze very small sample volumes.
Fast and reproducible results.
Suitable for both routine and research applications.
Limitations
Sensitive to the purity of the carrier gas.
Requires precise and stable calibration.
Cannot analyze non-volatile compounds without prior vaporization.
Conclusion
The Gas Chromatograph is a vital tool in analytical and biological chemistry. Its ability to accurately separate compounds, measure their concentrations, and integrate with advanced detectors like mass spectrometers makes it the instrument of choice in research, industrial, and medical laboratories.
If you want, I can also reformat this into a 10–12 slide PowerPoint presentation with diagrams for each GC component, ready for teaching or lab demonstrations.
Al-Mustaqbal University
Ranked First among Iraqi Private Universities