“A Scientific Article by the Head of the Department, Prof. Dr. Younis Abdul-Ridha Al-Khafaji” Artificial Kidneys: Between Implantation and Wearing... A Medical Revolution on the Horizon
Artificial Kidneys: Between Implantation and Wearing... A Medical Revolution on the Horizon
The Latest Scientific Developments
Research on artificial kidneys is witnessing remarkable progress that could bring about a qualitative shift in treating chronic kidney failure, which affects millions of patients worldwide. This research has taken two parallel paths: devices implantable inside the body and external wearable devices.
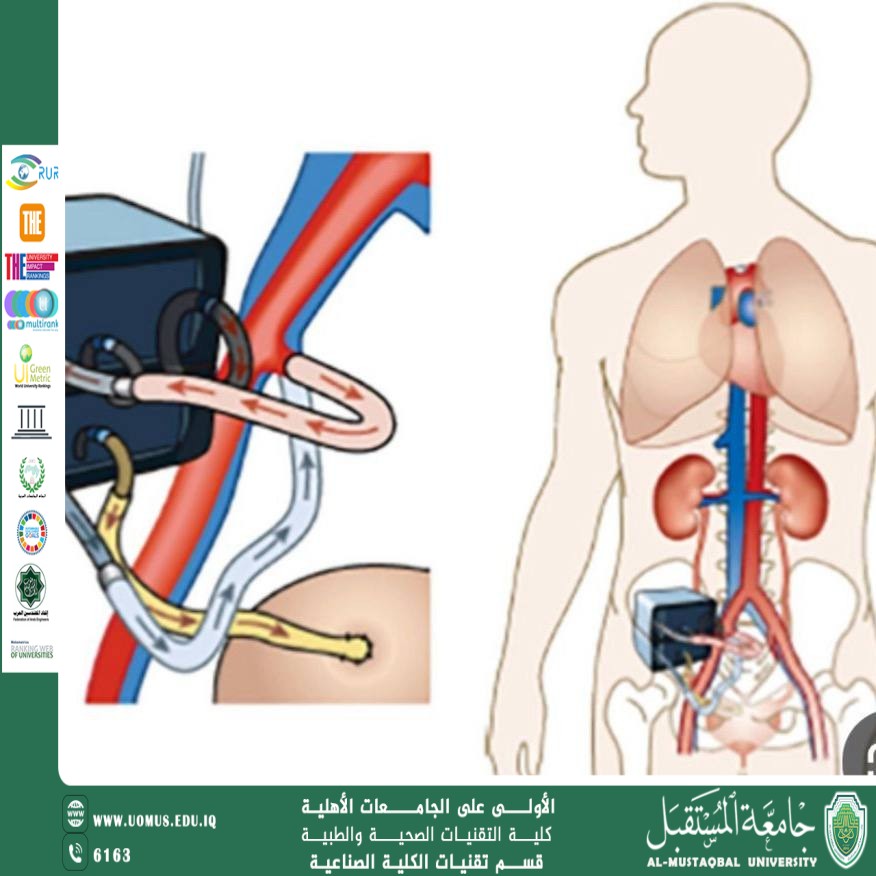
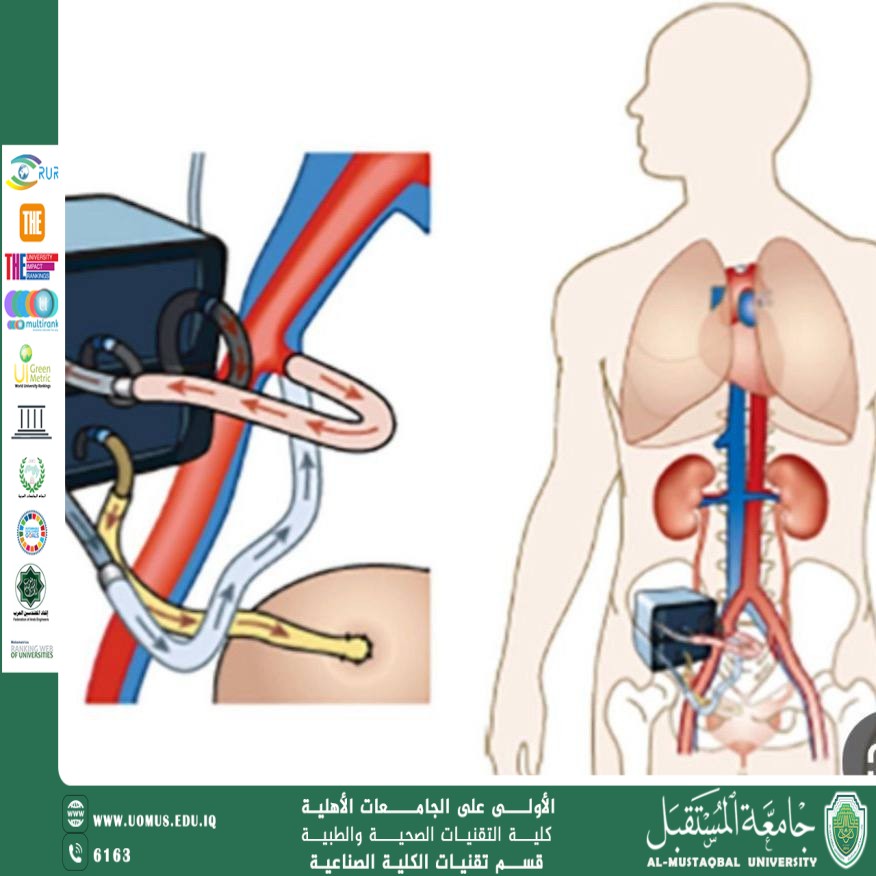
1. The Implantable Bioartificial Kidney
The latest advance in this field comes from "The Kidney Project" led by the Universty of California, San Francisco in collaboration with the National Institute of Biomedical Imaging and Bioengineering.
Technical Featurs:
A hybrid device combining a high-efficiency silicon filter and living kidney cells.
The device is about the size of a small coffee cup.
Operates using the patient's natural blood pressure without the need for external pumps.
Does not require immunosuppressive drugs.
https://via.placeholder.com/600x400/0047AB/FFFFFF?text=Bioartificial+Kidney+Implant
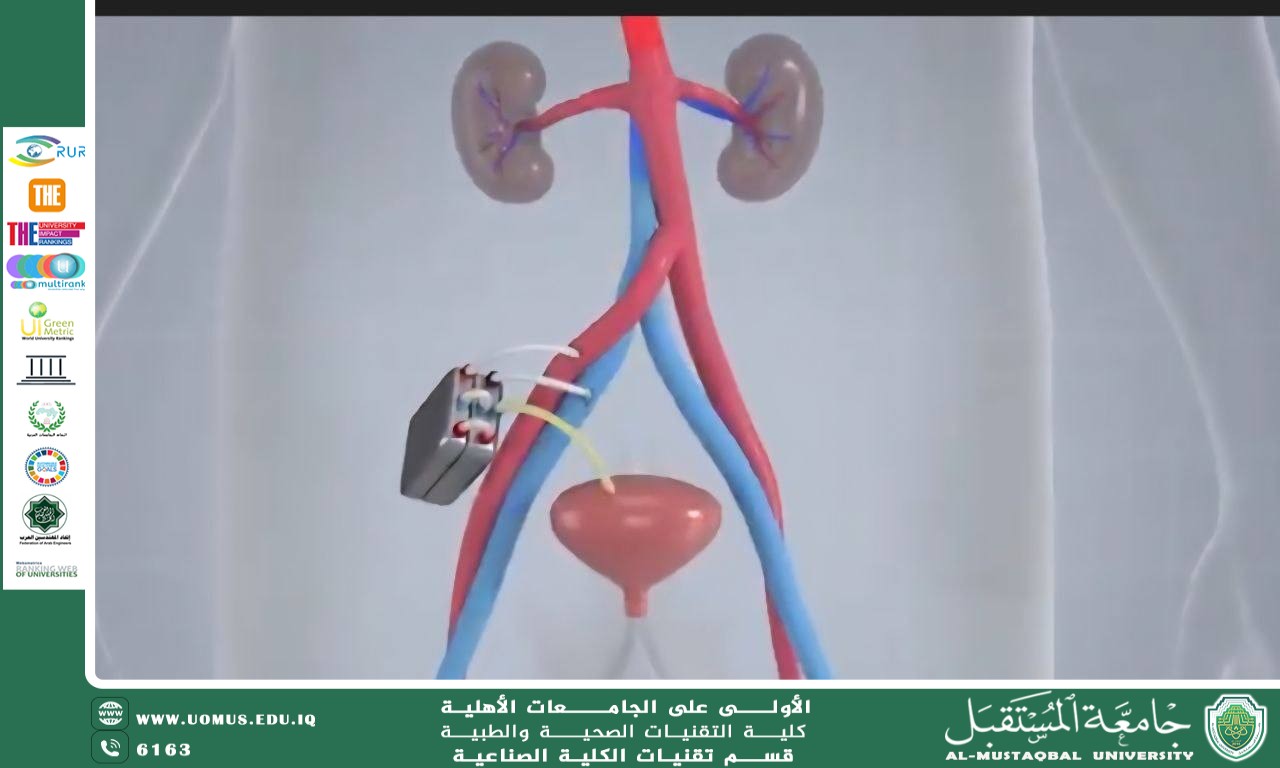
2. The Wearable Artificial Kidney
In the parallel path, the company "Aura" has developed a lightweight wearable device named "The Wearable Artificial Kidney (WAK)."
Device Specifications:
Weighs approximately 4.5 kilograms.
Operates on rechargeable batteries.
Provides continuous dialysis over 24 hours.
Gives patients freedom of movement and the ability to participate in daily activities.
https://via.placeholder.com/600x400/008000/FFFFFF?text=Wearable+Artificial+Kidney
The results of preliminary clinical applications on animals are highly encouraging, though still in their early and limited stages. No device has yet reached "large-scale clinical trials" (Phase 3) or obtained FDA approval for routine human use. However, preliminary data from early studies instill cautious optimism and prove the working principle of these devices.
Detailed Results by Main Project:
1. The Kidney Project - University of California, San Francisco (UCSF)
This is the most advanced project in the field of implantable devices.
Current Stage: Still in intensive preclinical trials on animals (especially pigs), which must be successfully completed before obtaining FDA clearance to begin human trials.
In late 2024, the team announced significant successes in:
Biocompatibility: The device does not cause significant blood clotting or acute immune response in animal models.
Filtration Function: The silicon filter (the first part of the device) efficiently filters blood.
Cell Viability: The living kidney cells (the second part of the device) remain alive and functional inside the implanted device for several weeks.
The goal is to continue improving the duration of cell function and preventing fibrosis around the device. The most realistic estimate for starting human clinical trials is 2026-2027.
2. The Wearable Artificial Kidney (WAK)
There are several prototypes, with the most developed being the one supported by Aura and the National Institute of Kidney's "Wearable Kidney" project.
Current Stage: Very early human clinical trials (First-in-Human and Phase 1). These are small trials (typically 10-15 patients) focusing on safety and feasibility, not long-term efficacy.
Early 2024 Results (announced at conferences like ASN Kidney Week):
Safety: The device was proven safe to wear for periods up to 24 hours in a controlled hospital environment. No serious adverse events (like severe bleeding or infection) directly linked to the device were reported.
Efficiency: The device effectively and continuously removed waste (like urea and creatinine) and excess fluid from the blood.
Patient Comfort: Participating patients reported significant improvement in symptoms they typically experience between traditional dialysis sessions, such as fatigue and muscle cramps.
Identified Challenges:
Mineral Precipitation: Risk of salt (calcium) deposition in the device tubing with prolonged use, potentially clogging it.
Electrolyte Balance: Need for precise and continuous monitoring of sodium and potassium balance in the blood, Engineering Design: Making the device lighter, quieter, and more reliable for home use.
Overall Assessment and Summary:
Aspect Current Status Is it Encouraging?
Proof of Concept Proven. The devices can filter blood and remove waste from the body. Yes, strongly. This is the foundation for all developments.
Short-Term Safety Encouraging in controlled environments. No major immediate risks identified in limited trials. Yes, cautiously.
Functional Efficacy Encouraging. Mimics kidney function better than intermittent traditional dialysis. Yes.
Readiness for Widespread Use Not ready. Technical (e.g., calcification) and regulatory challenges remain. No, but this is expected at this stage.
Timeline Slower than previously hoped. Technical complexity and regulatory rigor take time. Expectations must be adjusted, but progress continues.
Final Conclusion:
Yes, the results so far are generally encouraging. The technology has moved from a theoretical idea to working prototypes being tested on humans, with proof of the basic principle of safety and initial efficacy.
However, it is wise to temper expectations. 2025 is a year of intensive research and development, not a year of commercial availability. The remaining engineering and biological challenges are significant and require years of meticulous work to overcome.
Realistic Expectations:
Wearable Kidney: We may see larger clinical trials (Phase 2) in 2026, with the possibility of the first FDA-approved device by the end of the decade (2030).
Implantable Kidney: Animal trials will continue, with hope to begin human trials in 2026-2027 at the earliest.
In short, the ship has successfully sailed from port and proven its seaworthiness, but it is still on its journey across an ocean full of challenges before reaching the safe shore (patients in their homes).
Remaining Challenges and Future Prospects
Remaining Challenges:
Ensuring the long-term safety of implanted devices.
Preventing blood clotting inside the devices.
Reducing manufacturing costs.
Future Prospects:
Researchers plan to begin advanced human clinical trials in 2025.
Integration with AI technologies for function monitoring.
Developing more efficient purification systems using nanotechnology.
In conclusion, artificial kidneys represent real hope for kidney failure patients, approaching an unprecedented level of mimicking natural kidney function. With continued technical progress, we may witness a radical shift in kidney disease treatment over the next decade, granting patients longer lives and better quality of life.
Mustaqbal University
The First University in Iraq