Parkinson’s Disease: Discovery of a Protein That Creates Pores in Neuronal Cells Prepared by: Lect. Abbas Hamza Khudhair Department of Biochemistry – College of Science – Al-Mustaqbal University
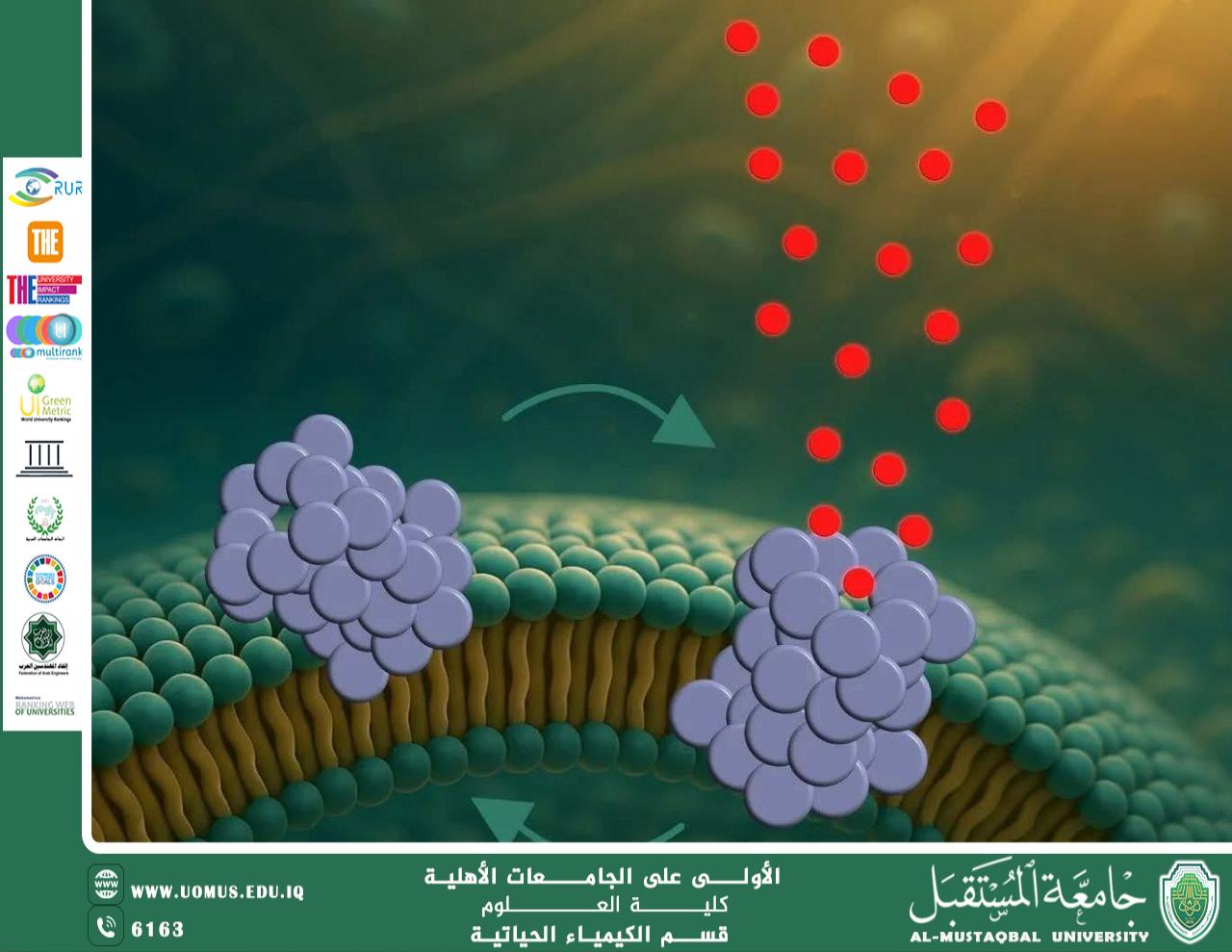
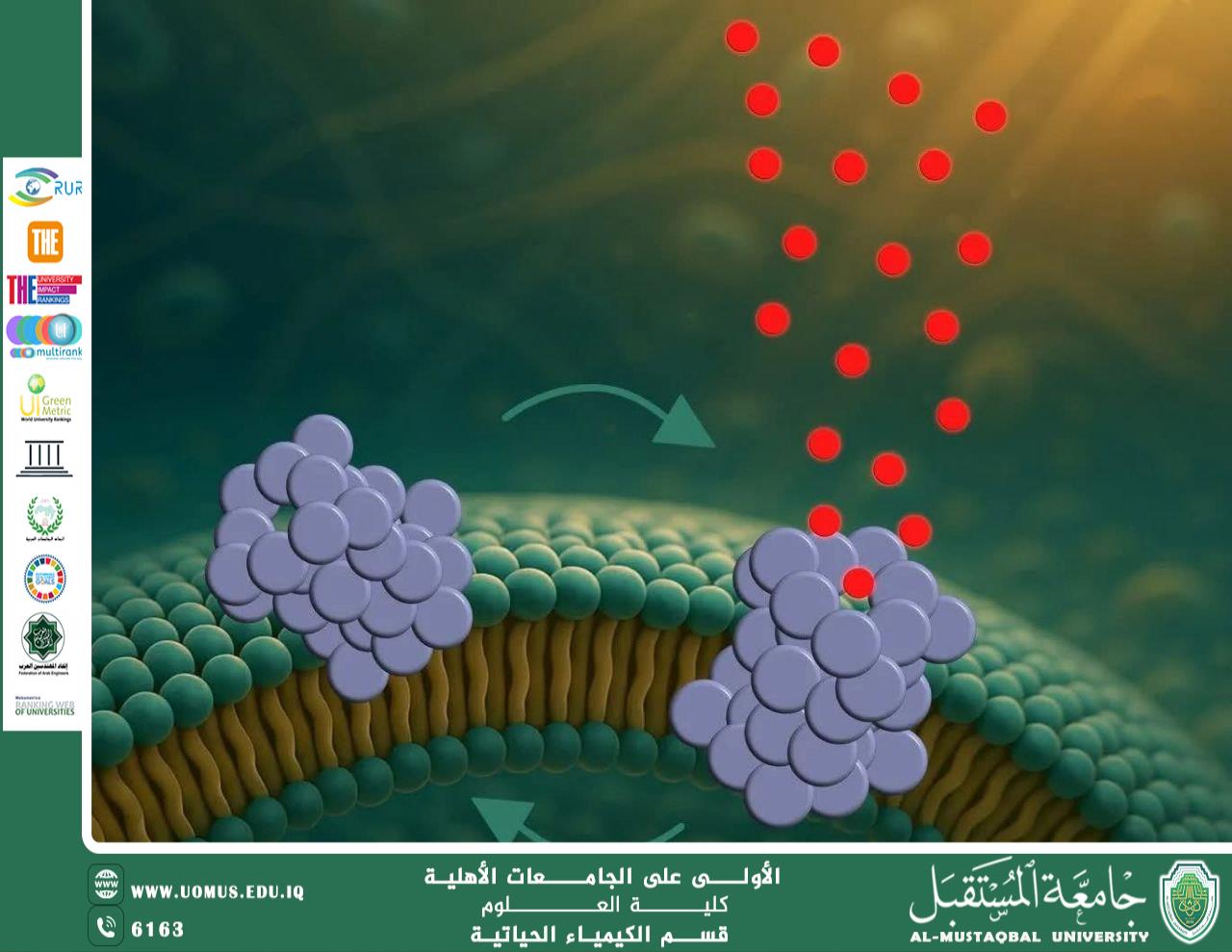
A recent study published in ACS Nano has revealed a previously unknown molecular mechanism that helps explain the progressive neuronal degeneration observed in Parkinson’s disease. The research demonstrates that small aggregates of alpha-synuclein (α-synuclein), known as oligomers, are capable of penetrating neuronal cell membranes and forming transient pores.
Mechanism of Membrane Damage
The neuronal cell membrane is a vital barrier that maintains intracellular chemical balance. The study shows that alpha-synuclein oligomers interact with the membrane through a three-step process:
They first bind to the membrane surface.
Then partially insert into the lipid bilayer.
Finally, they reorganize into pore-like structures that allow uncontrolled passage of molecules.
Remarkably, these pores are dynamic rather than permanent; they open and close stochastically, causing progressive and cumulative cellular damage instead of immediate cell death.
Pathological Significance
This dynamic pore formation helps explain the slow progression of Parkinson’s disease. A permanently open pore would kill the cell rapidly, but transient pores allow the cell to temporarily compensate before gradual failure occurs.
The study also indicates that highly curved membranes are particularly vulnerable, making mitochondria especially susceptible to damage. This provides a mechanistic link between mitochondrial dysfunction and neurodegeneration in Parkinson’s disease.
Therapeutic Implications
Nanobodies (single-domain antibody fragments) were tested and shown to successfully detect alpha-synuclein oligomers, but they did not prevent pore formation. These findings suggest that future therapeutic strategies should focus on preventing oligomer–membrane interactions or protecting cellular membranes.
Physiological Role of Alpha-Synuclein
Under normal conditions, alpha-synuclein is abundant in presynaptic nerve terminals and plays an important role in:
Regulating neurotransmitter release
Facilitating synaptic vesicle fusion with the plasma membrane
However, when the protein becomes misfolded, it loses its normal function and acquires toxic properties, contributing to neuronal death through a dual mechanism: loss of function and toxic gain of function.
Conclusion
This discovery represents a significant advance in understanding the molecular basis of Parkinson’s disease. By linking alpha-synuclein oligomers, membrane pore formation, and cellular homeostasis disruption, this study opens new perspectives for targeted therapeutic interventions aimed at preserving neuronal membrane integrity