Electrochemical Analysis in Analytical Chemistry
Introduction
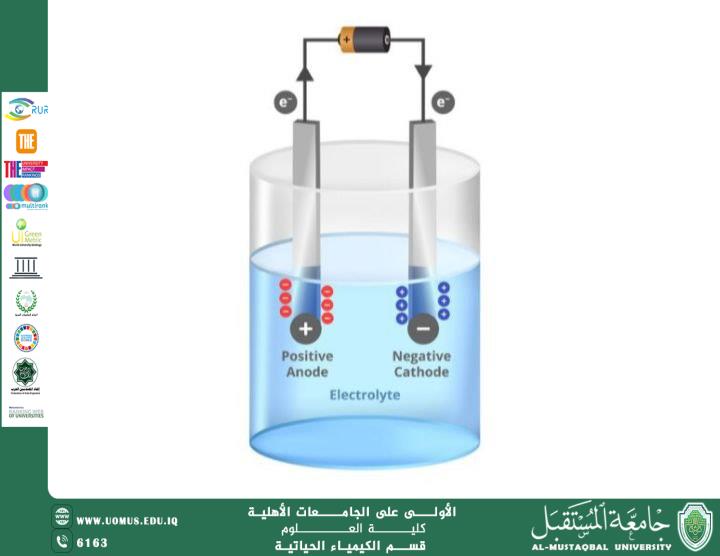
Electrochemical analysis is one of the modern techniques in analytical chemistry, relying on the study of chemical reactions that involve the transfer of electrons between electrodes. This type of analysis allows the determination of concentrations, the study of ion properties, and the analysis of chemical reactions with high accuracy. It is widely used in research, industrial, and medical laboratories, especially for detecting metals and ions in various samples.
Principle of Electrochemical Analysis
Electrochemical analysis is based on measuring the electrical changes resulting from oxidation-reduction (redox) reactions. This can be achieved in two main ways:
Direct measurement of current or potential generated by a specific reaction.
Quantitative monitoring of current or potential to determine the concentration of the analyte.
Instruments such as electrodes, electrochemical cells, and potentiostats are used to follow the reactions and analyze the data.
Types of Electrochemical Analysis
1️⃣ Potentiometry
Measures the electrical potential between two electrodes to determine ion concentrations.
Most common example: pH measurement using a glass electrode.
2️⃣ Amperometry
Measures the current produced by oxidation or reduction of a substance in solution.
Used to determine concentrations of certain ions or oxidizable compounds.
3️⃣ Voltammetry
Measures current while varying the applied potential gradually.
Used to determine concentrations of heavy metals and trace ions.
4️⃣ Polarography
A specialized form of voltammetry using a dropping mercury electrode.
Suitable for detecting metallic ions in aqueous solutions.
Applications of Electrochemical Analysis
Analysis of heavy metals in water and environmental samples.
Detection of metallic ions in food and pharmaceuticals.
Study of chemical reactions in research laboratories.
Monitoring industrial processes involving electrochemical reactions.
Advantages
High accuracy and excellent sensitivity for ions.
Can analyze very small amounts of substances.
Allows simultaneous qualitative and quantitative analysis.
Disadvantages
Requires specialized equipment and precise calibration.
Some analyses require special sample preparation.
Results may be affected by chemical and physical factors such as temperature and conductivity.
Conclusion
Electrochemical analysis is a vital tool in modern analytical chemistry, providing accurate and reliable measurements of ion concentrations and chemical reaction properties. This technique is widely applied in research, industrial, and medical laboratories for the detection and analysis of metals and ions.