yThe Role of Spectroscopic Analysis in Verifying the Purity of Compounds Dr. Karrar Majid Ubaid Department of Analytical Chemistr
In the field of analytical chemistry, verifying the purity of chemical compounds is one of the fundamental tasks that ensures the quality of materials used in research and industrial applications. Spectroscopic analysis techniques are considered essential tools for determining compound purity, as they provide accurate and comprehensive information about the chemical composition of the material under investigation.
What Is Spectroscopic Analysis?
Spectroscopic analysis is a technique based on studying the interaction between electromagnetic radiation and matter. Spectra are obtained by measuring the absorption, emission, or scattering of light or radiation by a sample in order to analyze its properties. Spectroscopic techniques vary depending on the type of radiation used, such as visible light, ultraviolet radiation, infrared radiation, or X-rays.
Types of Spectroscopic Techniques
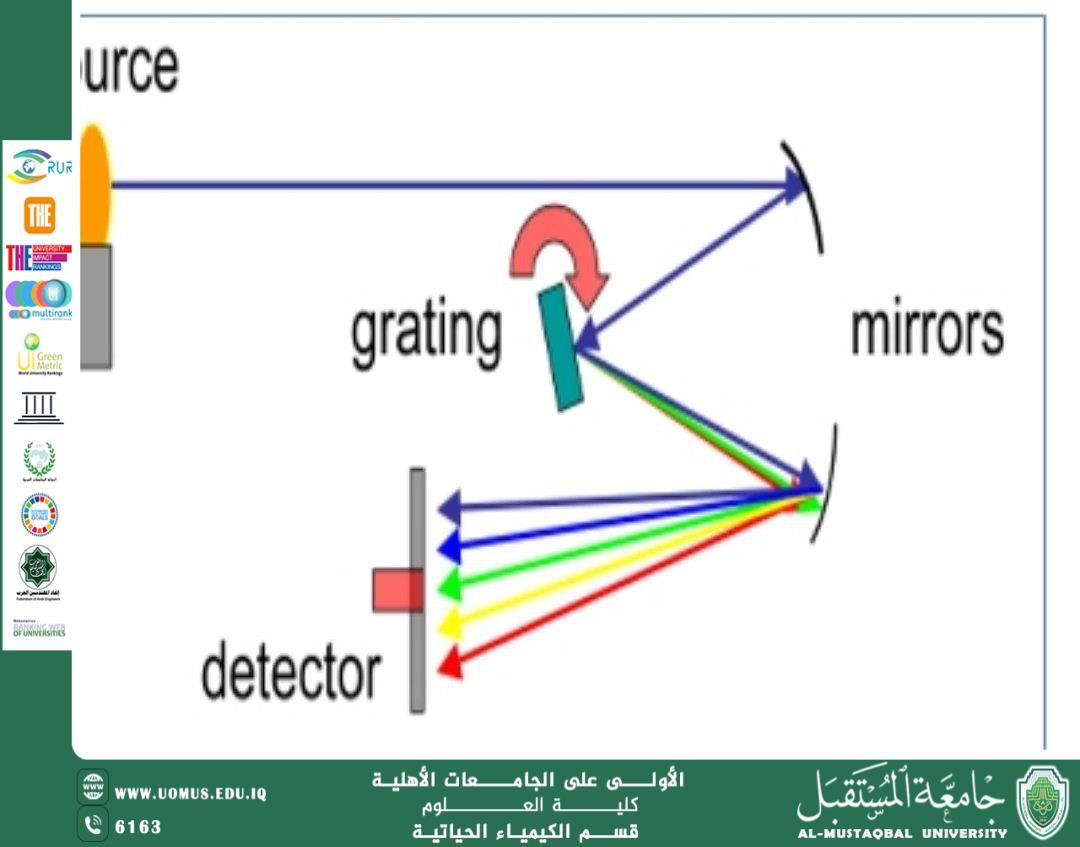
1. Ultraviolet–Visible Spectroscopy (UV–Vis)
This technique is used to measure the absorption of light in the ultraviolet and visible regions. It relies on the ability of compounds to absorb light at specific wavelengths, which helps identify the presence of impurities or additional compounds in the sample.
Role in Purity Verification:
By comparing the obtained UV–Vis spectrum with that of a pure reference compound, any deviations or unexpected absorption peaks may indicate the presence of impurities.
2. Infrared Spectroscopy (FTIR)
FTIR spectroscopy is used to analyze molecular vibrational frequencies and is particularly effective for identifying functional groups in organic and inorganic compounds.
Role in Purity Verification:
FTIR helps confirm the presence of expected functional groups and detect any additional peaks resulting from impurities or unwanted side reactions.
3. Nuclear Magnetic Resonance Spectroscopy (NMR)
NMR is a powerful technique for analyzing the molecular structure of organic compounds, providing detailed information about the chemical environment of atoms within a molecule.
Role in Purity Verification:
NMR can detect contamination, side products, or incomplete reactions, ensuring the integrity and purity of the compound’s chemical structure.
4. X-ray Spectroscopy
X-ray techniques are used to study crystal structures and determine the crystalline arrangement of compounds.
Role in Purity Verification:
For crystalline compounds, X-ray analysis can reveal structural irregularities or foreign phases, indicating the presence of impurities within the crystal lattice.
Importance of Spectroscopic Analysis in Purity Verification
Accuracy and Reliability:
Spectroscopic techniques provide highly precise information about chemical composition and molecular structure, ensuring reliable verification of compound purity.
Detection of Impurities:
In pharmaceutical and chemical industries, impurities can significantly affect product safety and performance. Spectroscopy is an ideal tool for detecting such impurities at various stages of production.
Analysis of Complex Samples:
Spectroscopic methods enable the analysis of complex mixtures, allowing identification of the main compound without extensive sample preparation.
Time and Effort Efficiency:
Compared to traditional analytical methods, spectroscopic techniques are faster and more efficient, accelerating the purity verification process.
Conclusion
Spectroscopic analysis techniques play a vital role in ensuring the purity of chemical compounds for both research and industrial purposes. By utilizing these advanced tools, scientists and engineers can confirm that the materials they use meet the highest standards of quality and purity.
With continuous advancements in spectroscopic technologies, more complex analyses can be performed with greater accuracy and lower cost, contributing to improved production processes and enhanced product safety.
Al-Mustaqbal University
Ranked First Among Private Iraqi Universities
إ