A Scientific Article By Prof. Dr. Mahdi Abdulkadhim Abed Entitled:Some Thermodynamic Criteria in Saline water
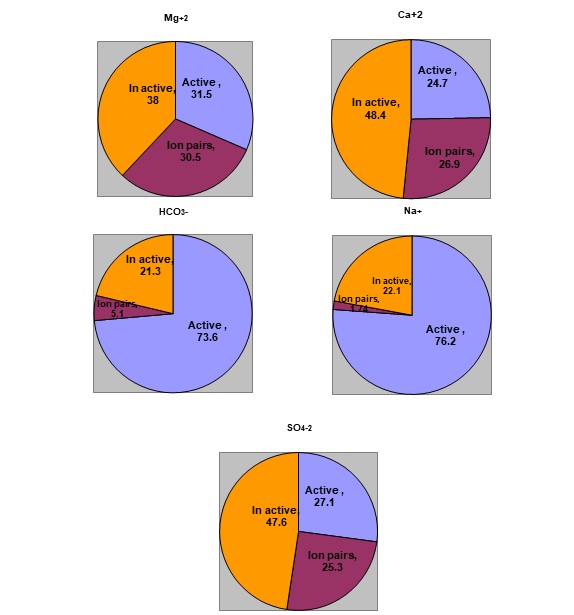
<br /><br />Prof. Dr. Mahdi Abdulkadhim Abed <br /><br /> Chemical complexes in natural water can be divided mainly into three groups: ionic pairs of the main components, inorganic complexes, and organic complexes. The first group is of great importance in the chemistry of water and solutions with high ionic strength. Therefore, the management of water and reclaimed soil requires good knowledge about the chemical properties of the solution. Soil and water, especially dissolved positive and negative ions, and one of the important topics is the interaction between these ions and the formation of the ion pairs. When salts are dissolved in water, they usually decompose completely, but this is not a general rule. For example, a large portion of the positive and negative ions of some strong electrolytes are attracted to each other at a distance of less than 5Å , and then the force of attraction exceeds the force of repulsion and are connected by Bonds thus lose their separate properties. As a result of this bonding, while each ion remains with its aqueous layer and behaves as a single ion on top of it, this phenomenon is called ion pairs..<br /> The figures show the percentages of ion pairs and the active and inactive forms for each of calcium, magnesium, sodium, sulfate, and bicarbonate. It is noted that the activety of calcium and sulfate ions is low compared to the activety of sodium ,bicarbonate ions and this is attributed to the participation of a large portion of calcium, magnesium, and sulfate ions, while the activety of sodium and bicarbonate is high due to the low of ions pairs, and this reflects the effect of sodium ions on the chemical and physicochemical properties..<br /><br /><br /><br /><br /><br /><br />