Nitriding
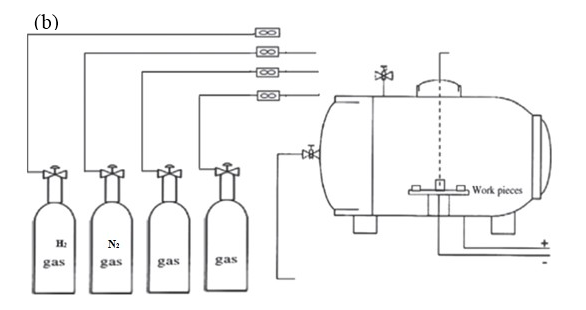
The chief application of surface engineering is to create a solid layer on a tough material surface to enhance wear resistance. The base nitride is atomic nitrogen diffused on the steel surface, where it is in state of ferritic.<br /> Nitriding is related to nitrogen for iron. At high temperatures, the lattice of BCC ferritic iron starts to vibrate. These make the atom of nitrogen vibrate and diffuse through lattice of iron and form nitrides of iron or other alloying elements such as Cr, V, and Ti. The nitride preparation of elements such as Cr2N, Al N, VN, Mo2N, and W2N can lead to an interlock of slip planes with the consequent hardening of the case.<br />1. Nitriding Surface Layers Formation:<br /> The surface treatment of nitrogen involves two regions:<br />1. Layer Compound (White) <br /> The top layer thickness highly depends on chemical composition. For example, for plain carbon steel, the layer compound thickness is more in contrast with steel alloy. The layer compound is primarily formed with two iron nitrides of γ -Fe4N and γ-Fe2-3N.<br /> The area where the content of nitrogen on the surface is more, the formation of γ-Fe2-3N accelerates. On the other hand, with an increment in the distance from the surface to the core, the γ-Fe2-3N phase is replaced with γ-Fe4N and its quantity decreases correspondingly.<br /> A compound layer of predominately ɛ phase will create on a surface with good wear characteristics, but it will have no impact strength. The presence of γ -Fe4N can enhance the layer impact force.<br /><br /><br />2. Diffusion Layer <br /> A diffusion layer under the compound layer uses nitriding for alloying elements. When compared with the compound layer, it can affect fatigue and the hardness of a substrate. An increase in the hardness of nitriding of steel is seen because of the presence and distribution of alloying elements nitrided for elements of alloying. However, by compound layer preparation, the nitrogen and conglomerate by nitride-forming elements can be diffused with the formation of nitriding compounds. Those nitrides were precipitated on the grain boundaries, which contribute to the increment in the diffusion layer hardness.<br />2. Nitriding Methods: <br />1. Liquid Nitriding.<br /> The process of liquid nitriding takes place in a molten salt bath comprising either cyanates or cyanides at a temperature of 510-580°C. The nitrogen diffusion is faster compared with carbon due to low applied temperature; therefore, carburizing does not occur. Sodium salts have a general composition of 60-70% and salts of potassium 30-40 %. This work led to a decrease in quantity of carbonate and cyanide. However, the treatment of nitriding typically can be conducted in a range of temperatures (540-595°C) using the characterics of the nitride layer, which depend on cyanate to cyanide ratio plus the quantity of existing cyanate in the bath.<br />2. Gas Nitriding.<br /> The temperature of nitriding falls in the range of 500-565°C and under Ac1 (the temperature of austenite formation of ferritic steel). Before attaining the temperature of 150°C, furnace should be vacuum to protect the samples from oxidation. Dry ammonia is puffed into the furnace and decomposed over the specimen’s surface. After this, as an effect of reactions by elements of alloying, different nitrides are formed. Atomic nitrogen is adsorbed on the surface of steel.<br />3. Plasma Nitriding. <br /> Plasma is broadly used for surface treatment such as, deposition of vapor, spraying, and nitriding. It is prepared by ionizing the atoms of the gas through discharge of electrical or radiation of heat. Plasma is a leading gas and contains a noticeable proportion of charged particles. In plasma nitriding, the nitrogen is presented to the surface of the substrate when being diffused with the metal. <br /> 3.The following parameters can be influenced by the process of plasma nitriding: <br />1.Gas Mixture:<br /> The general gas mixture used for plasma nitriding is 25% N2 + 75% H2. If the nitrogen rises to 50%, the growth rate increases. Furthermore, an increase in nitrogen can lead to compound layer thickening. There are varying opinions concerning the role of hydrogen in the process of nitriding. Some researchers consider it to play a positive role, such as increase in the thickness of the nitrided case, reaction rate, and active nitriding density in the plasma. Several researchers have also concluded hydrogen has no deleterious effect.<br />2.Temperature:<br /> The temperature of plasma nitriding plays an important role along with the depth of nitriding and must be about of 350–580°C. Steel alloy requires lesser temperature than carbon steel. Pressure, time, and the power of plasma are other parameters that can affect the condition of plasma nitriding.<br />4. Benefits and Disadvantages of Plasma Nitriding:<br /> The benefits of the plasma nitriding are as follows:<br />1. Decrease the time of processing.<br />2. Decrease distortion plus dimensional changes.<br />3. No need to process the final finishing.<br />4. Uniformity and homogeneity for the surface of samples plus the geometry of the complex.<br />5. Harmless for plasma nitriding.<br />6. Reaction of quick kinetic on the surface.<br />7. Ability to eliminate oxide films before nitriding for nitrogen mass transfer acceleration from plasma to the component.<br />