Nutritional Modulation of the Central Nervous System and Immune Homeostasis
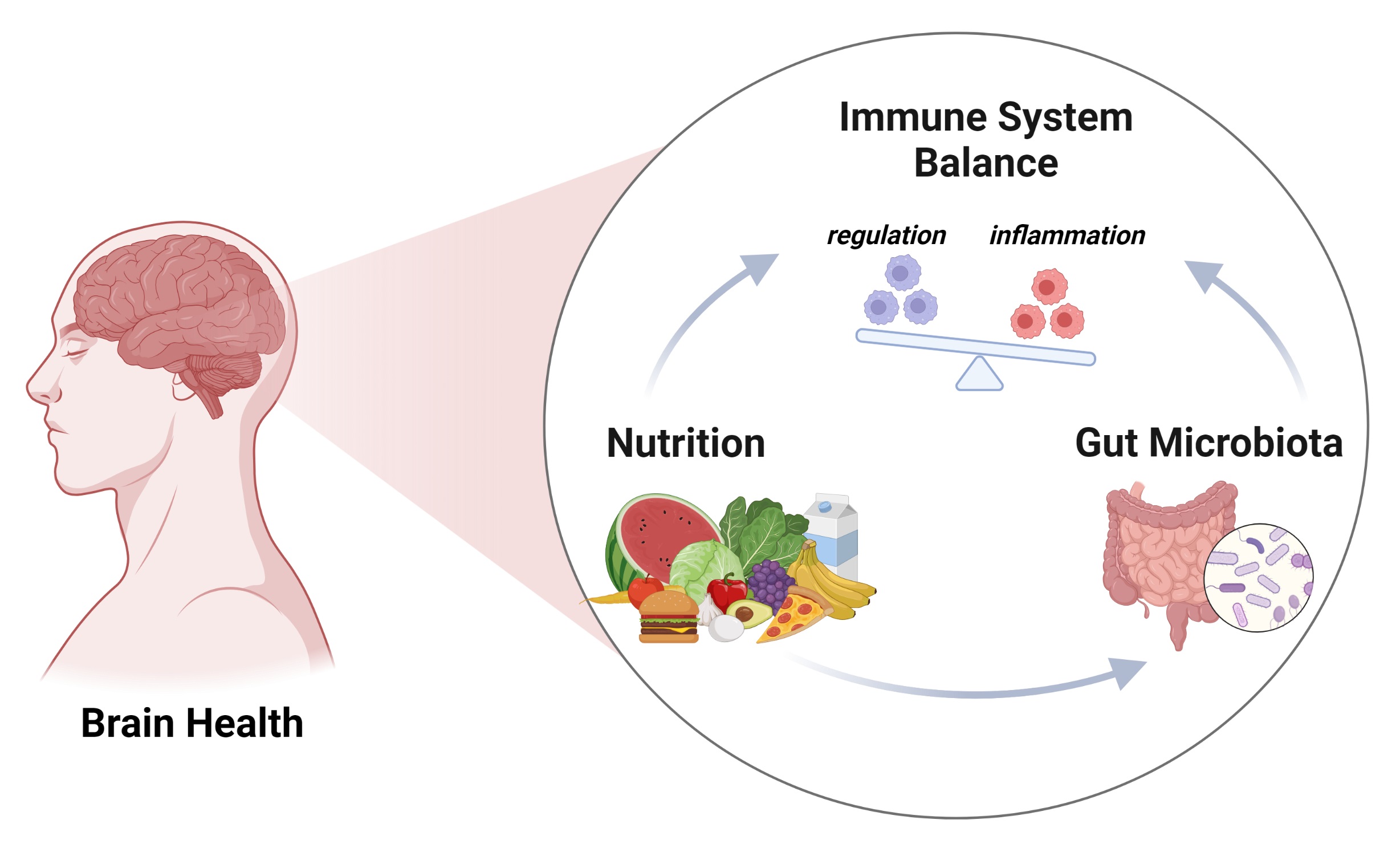
The role of diet in the development of metabolic alterations leading to chronic inflammatory disease is becoming increasingly clear, owing to extensive research on the subject in recent years. There is ample evidence connecting metabolic disease, such as obesity and and type 2 diabetes mellitus (T2DM), to dietary changes, including increased intake of high-calorie content, highly processed food and their constituent additives, as well as decreased consumption of fruits, vegetables, and other sources of dietary fiber, common characteristics of what is known as “western” diets. Metabolic changes characterized by impaired glucose metabolism and insulin resistance, leading to alterations in systemic energy availability, chronic low-grade inflammatory responses, and increased oxidative stress, are hallmarks of metabolic dysregulation observed in obese and diabetic patients and are also known factors involved in development of neurological pathologies including Alzheimer’s (AD), Parkinson’s (PD), and Huntington’s (HD) diseases. <br /> High body-mass index (25–30 or above) is related to two- to three-fold increased risk for developing dementia in humans and this outcome has been attributed primarily to the effects of hyperglycemia and impaired insulin-dependent signaling which promote inflammation and oxidative stress both systemically and in the central nervous system (CNS), leading to neuronal and glial damage and development of neurodegenerative pathologies. Furthermore, chronic metabolic dysregulation increases the risk of cognitive impairment and dementia in patients with diabetes, due to enhanced glucose-dependent oxidative stress, presence of advanced glycation end products, inflammation and atrophy of brain regions like the hippocampus and amygdala.<br /> The Gut-Microbiome-Brain Axis<br />The human microbiome comprises a variety of microorganisms from multiple phyla, inhabiting the skin and mucosal tissues .The intestinal microbiota, in particular, is comprised mainly by five different phyla and multiple genera of the Eubacteria domain: Actinobacteria (Bifidobacterium), Bacteroidetes (Bacteroides and Prevotella), Firmicutes (Clostridium and Lactobacillus), Proteobacteria (Escherichia), and Verrucomicrobia (Akkermansia), which present significant variability among individuals and populations, with some estimates suggesting over 1000 bacterial species that may be present within specific populations .The relevance of the microbiome for the modulation of metabolism has become evident with studies on biochemical parameters related to glucose tolerance and insulin production in germ-free mice with further evidence emerging from studies on changes in the susceptibility to obesity in genetically obese and lean animals with altered microbiomes. Presently, the microbiome is known to participate in the regulation of multiple physiological processes, including energy balance and the development and functions of the immune system, with alterations in its composition and functions modifying the development and evolution of multiple pathologies .The microbiome in the gastrointestinal system is an essential component of systemic metabolism, as the bacteria it contains plays an important role in nutrient absorption and synthesis of multiple metabolites relevant for human health, including lipids, amino acids, vitamins, bile acids, and short-chain fatty acids (SCFAs), as well as bacteria-specific products including peptidoglycans and lipopolysaccharides. The role of the gut microbiome in energy metabolism in humans is still being elucidated. It has been suggested that specific bacterial genera are responsive to energy availability in the organism or to the calorie content in diet, as obese mice and humans present an increased amounts of Firmicutes and decreased amounts of Bacteroidetes in the intestine, and these levels are reversed when the animals are fed a calorie-restricted diet or when weight is lost, either by reducing calorie intake or by surgical procedures . In addition, germ-free mice that are reconstituted with bacteria from obese animals gain more weight and adipose tissue than those reconstituted with bacteria from lean animals, suggesting that the intestinal microbiome is relevant for regulation of energy metabolism in the host. <br /> In addition to its regulatory effects on metabolism, the intestinal microbiome plays an essential role in the modulation of immune and nervous system functions, either through direct production of bioactive components affecting the release of hormones, incretins and neurotransmitters, or by regulation of leukocyte functions, such as cytokine production .Therefore, the microbiome is part of a complex bidirectional communication system integrating the gastrointestinal, immune, and nervous systems and its stability and adequate functional status is necessary for maintenance of human health.<br />د.زهراء طارق حسون <br />دكتوراه فسلجة الدماغ والاعصاب<br />