Color Change with pH: A Study of pH Indicators and Their Applications
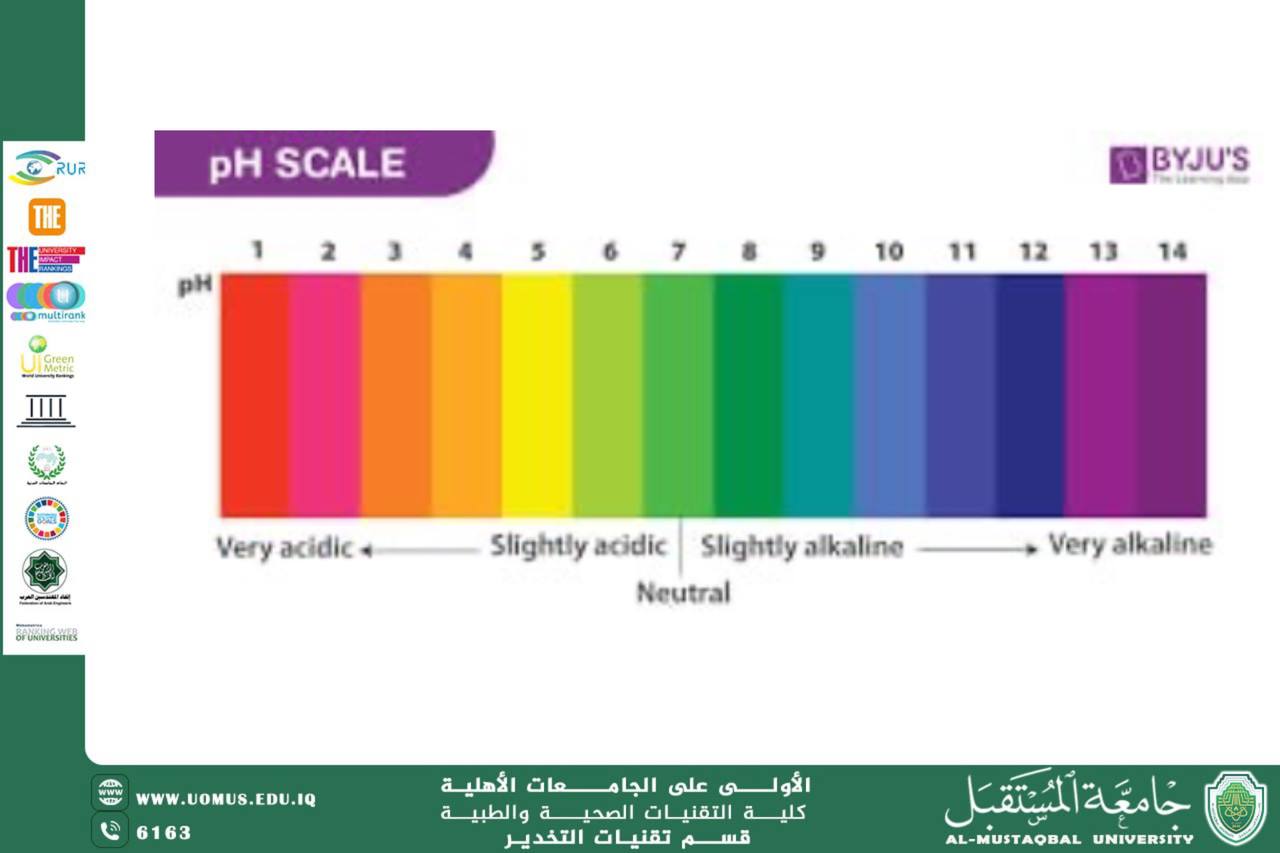
The pH level of a solution is a measure of its acidity or alkalinity. It is crucial in many chemical processes, biological systems, and industrial applications. One of the interesting phenomena related to pH changes is the alteration in the color of certain substances. This color change, which occurs in response to variations in pH, is used in a variety of applications, such as in pH indicators, biochemical research, and environmental monitoring.<br /><br />What Causes Color Change with pH?<br />The color change that occurs with varying pH is primarily due to the presence of pH indicators—substances that exhibit different colors depending on the acidity or basicity of a solution. These indicators are typically weak acids or bases, which undergo chemical reactions in response to changes in pH.<br /><br />A pH indicator works by dissociating into different ions at different pH levels. The equilibrium between these forms is influenced by the concentration of hydrogen ions (H⁺) or hydroxide ions (OH⁻) in the solution. When the pH changes, the equilibrium shifts, causing a change in the color of the indicator. This is because the ionization state of the indicator affects its molecular structure, which in turn alters how it absorbs and reflects light.<br /><br />Common pH Indicators and Their Color Changes<br />Litmus: Litmus is one of the most widely recognized pH indicators. It turns red in acidic conditions (pH < 7) and blue in basic conditions (pH > 7). This is due to a structural change in the litmus molecule as it reacts with hydrogen ions in acidic environments.<br /><br />Phenolphthalein: Phenolphthalein is commonly used in titrations and is colorless in acidic solutions. As the pH increases and the solution becomes more basic (pH > 8.3), phenolphthalein undergoes a change and turns pink. The color intensity increases with higher pH values.<br /><br />Methyl Orange: Methyl orange is another useful pH indicator, particularly in titrations involving strong acids and bases. It changes from red in acidic conditions (pH < 3.4) to yellow in basic conditions (pH > 4.4). This transition occurs due to the protonation and deprotonation of the indicator molecule.<br /><br />Bromothymol Blue: Bromothymol blue is used to detect changes in pH between 6.0 and 7.6. It is yellow in acidic solutions and blue in basic solutions, with a greenish color at neutral pH (around 7).<br /><br />Universal Indicator: A universal indicator is a mixture of several pH indicators that exhibit a range of colors across the entire pH scale. The color changes gradually from red at a very low pH (acidic) to green at neutral pH, and to blue or violet at higher pH values (alkaline).<br /><br />Mechanism Behind the Color Change<br />The color change observed in pH indicators is due to the protonation or deprotonation of specific functional groups within the indicator molecule. For instance:<br /><br />In acidic conditions, the concentration of H⁺ ions is high, causing the indicator molecule to gain protons (H⁺), which leads to a structural change that produces a specific color.<br />In basic conditions, where there is an excess of OH⁻ ions and a low concentration of H⁺, the indicator may lose protons, leading to a different structural form with a distinct color.<br />This protonation-deprotonation process is reversible, meaning that the color can shift back and forth if the pH fluctuates.<br /><br />Applications of pH-Dependent Color Change<br />Titration: The color change of pH indicators is most commonly used in acid-base titrations to determine the concentration of an unknown acid or base. By adding the titrant to a solution and observing the point at which the indicator changes color, the equivalence point of the reaction (where the amount of acid equals the amount of base) can be identified.<br /><br />Biological Systems: In biological systems, the pH of bodily fluids (such as blood and urine) can affect the activity of enzymes and metabolic processes. pH indicators are used in laboratory tests to monitor these fluids and to ensure that they maintain the proper pH for normal physiological function.Environmental Monitoring: pH indicators are crucial in environmental science for monitoring the health of water bodies. Changes in pH levels in oceans, lakes, and rivers can indicate pollution, acid rain, or other environmental stresses. The color changes observed with pH indicators provide a simple way to assess water quality.<br /><br />Food Industry: pH indicators are also used in the food industry to monitor the acidity of food products. The pH of foods affects their taste, texture, and preservation. For example, in cheese making or fermentation processes, maintaining the correct pH is essential for product quality.<br /><br />Home Testing Kits: Many home testing kits for substances like pool water, soil, or even urine include pH indicators. These indicators change color to give a rough estimate of the pH of the sample, helping individuals make informed decisions about their environment or health.<br /><br />Conclusion<br />The phenomenon of color change with pH is not only a fascinating chemical process but also a highly practical tool in various fields of science and industry. pH indicators, through their ability to visibly signal changes in acidity or alkalinity, have become indispensable in fields ranging from chemical analysis and environmental monitoring to healthcare and food production. By understanding the underlying principles of these indicators and their color changes, we can continue to use them effectively in diverse applications, improving our ability to measure, monitor, and maintain balance in both natural and industrial systems.<br />م.م زينب عباس مالك<br /><br />AL_mustaqbal University is the first university in Iraq<br />