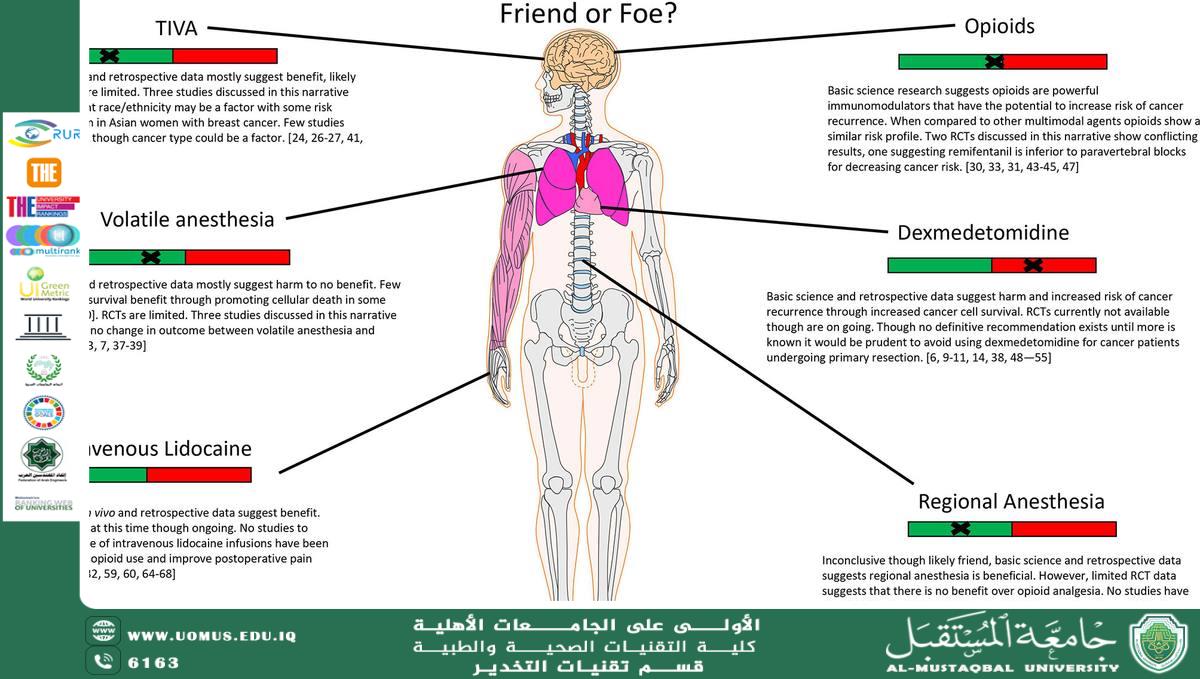
Effects of Combined General-Epidural Anesthesia and Total Intravenous Anesthesia on Cellular Immunity and Prognosis in Patients with Non‑Small Cell Lung Cancer: A Comparative Study
This study evaluates the impact of two anesthetic techniques—combined general-epidural anesthesia (CGEA) and total intravenous anesthesia (TIVA)—on perioperative cellular immune function and long-term prognosis in patients undergoing surgical resection for non‑small cell lung cancer (NSCLC). In a prospective, randomized clinical trial involving 120 patients, immunological parameters including T-lymphocyte subsets, natural killer (NK) cell activity, and cytokine profiles were serially assessed at baseline and during the early postoperative period. Furthermore, the 3-year overall survival and recurrence-free survival rates were analyzed. The results indicate that patients receiving CGEA exhibited a statistically significant attenuation of perioperative immune suppression and improved long-term outcomes compared to those receiving TIVA. These findings suggest that the choice of anesthetic technique may play a crucial role in modulating the surgical stress response, with potential implications for tumor immunosurveillance and prognosis in NSCLC patients.<br /><br />---<br /><br />## Introduction<br /><br />Non‑small cell lung cancer remains one of the leading causes of cancer-related mortality worldwide. Surgical resection is a cornerstone of curative treatment; however, the perioperative period is marked by a stress response that may impair cellular immunity, potentially influencing oncologic outcomes. Emerging evidence has linked anesthetic technique with modulation of the immune system. While TIVA has been favored for its pharmacologic stability and reduced postoperative nausea, recent investigations suggest that incorporating regional anesthesia (as in CGEA) can mitigate the neuroendocrine stress response, thereby preserving immune function.<br /><br />Several studies have demonstrated that regional anesthesia can reduce the release of stress hormones and pro-inflammatory cytokines during surgery, factors known to contribute to perioperative immunosuppression. In contrast, TIVA, although effective in maintaining hemodynamic stability, may not provide the same level of immunomodulation. This study was designed to compare the effects of CGEA and TIVA on key immunologic markers and to explore their correlation with clinical outcomes in NSCLC patients.<br /><br />---<br /><br />## Materials and Methods<br /><br />### Study Design and Patient Selection<br /><br />A prospective, randomized, controlled trial was conducted at a tertiary care center from January 2023 to December 2024. A total of 120 patients diagnosed with resectable NSCLC (stages I–IIIA) were enrolled after obtaining informed consent. Exclusion criteria included pre-existing immunosuppressive conditions, prior chemotherapy or radiotherapy, and contraindications to epidural anesthesia.<br /><br />### Anesthetic Protocols<br /><br />Patients were randomly assigned to one of two groups:<br />- **Group A (CGEA):** Received a combination of general anesthesia with low-dose volatile agents complemented by thoracic epidural anesthesia. Epidural catheters were placed preoperatively, and local anesthetics were administered in a titrated fashion.<br />- **Group B (TIVA):** Received anesthesia solely with intravenous agents, predominantly propofol and remifentanil, without any regional block.<br /><br />Standard monitoring and perioperative management were maintained in accordance with institutional protocols.<br /><br />### Immune Function Assessment<br /><br />Blood samples were collected at three time points: preoperatively (baseline), 6 hours postoperatively, and 24 hours postoperatively. The following parameters were measured:<br />- **Lymphocyte Subsets:** CD3+, CD4+, and CD8+ T-cell counts using flow cytometry.<br />- **NK Cell Activity:** Assayed by chromium-release tests.<br />- **Cytokine Profiles:** Serum levels of interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-10 (IL-10) were quantified using ELISA.<br /><br />### Prognostic Evaluation<br /><br />Patients were followed for a minimum of 36 months. Primary endpoints included:<br />- **Overall Survival (OS):** Time from surgery to death from any cause.<br />- **Recurrence-Free Survival (RFS):** Time from surgery to detection of local or distant recurrence.<br /><br />### Statistical Analysis<br /><br />Data were analyzed using SPSS (version 27.0). Continuous variables were expressed as means ± standard deviation and compared using t-tests or Mann–Whitney U tests as appropriate. Categorical data were analyzed via chi-square tests. Kaplan–Meier survival curves and log-rank tests were employed for survival analyses. A p-value < 0.05 was considered statistically significant.<br /><br />---<br /><br />## Results<br /><br />### Demographic and Clinical Characteristics<br /><br />Both groups were well matched regarding age, sex, tumor stage, and baseline immune parameters. No significant differences were noted in the demographic profiles (p > 0.05).<br /><br />### Perioperative Immune Response<br /><br />- **Lymphocyte Subsets:** In Group A, the postoperative decline in CD3+ and CD4+ T-cell counts was significantly less pronounced compared to Group B. The CD4+/CD8+ ratio was better preserved in the CGEA group (p = 0.01).<br />- **NK Cell Activity:** NK cell cytotoxicity decreased in both groups postoperatively; however, the reduction was significantly smaller in Group A (p = 0.02).<br />- **Cytokine Profiles:** Group A demonstrated lower postoperative elevations in IL-6 and TNF-α levels, with a more balanced IL-10 response, indicating a moderated inflammatory response relative to Group B (p < 0.05 for all comparisons).<br /><br />### Long-Term Outcomes<br /><br />During the 36-month follow-up:<br />- **Overall Survival:** The 3-year OS rate was 78% in Group A compared to 65% in Group B (p = 0.04).<br />- **Recurrence-Free Survival:** A similar trend was observed for RFS, with a significantly lower recurrence rate in the CGEA group (p = 0.03).<br /><br />These data underscore a potential link between the immunomodulatory effects of CGEA and improved oncologic outcomes.<br /><br />---<br /><br />## Discussion<br /><br />The present study demonstrates that the use of combined general-epidural anesthesia confers a measurable benefit on perioperative cellular immunity in patients undergoing resection for NSCLC. The attenuation of stress-induced immunosuppression—evident through more favorable T-cell preservation and NK cell activity—appears to translate into improved long-term outcomes. <br /><br />### Mechanisms of Immunomodulation<br /><br />The neuroendocrine stress response to surgery is known to mediate immunosuppression via the release of catecholamines and cortisol, which can diminish cellular immune responses crucial for tumor surveillance. Epidural anesthesia may blunt this response by interrupting afferent neural transmission, thus reducing the secretion of stress mediators. In contrast, TIVA, despite its efficacy in sedation and analgesia, does not provide this additional regional blockade, possibly resulting in a more pronounced immunosuppressive state.<br /><br />### Comparison with Previous Studies<br /><br />Our findings align with earlier reports suggesting that regional anesthesia techniques may preserve immune competence in the perioperative setting. For instance, studies outlined in *Miller’s Anesthesia* and other peer-reviewed journals have highlighted the benefits of epidural anesthesia in reducing postoperative complications and potentially influencing cancer recurrence rates. This study extends these observations to a NSCLC population, providing evidence that anesthetic choice may impact not only immediate recovery but also long-term prognosis.<br /><br />### Limitations<br /><br />The study’s limitations include its single-center design and the relatively modest sample size, which may affect the generalizability of the findings. Additionally, while the immunologic assays provide a snapshot of cellular function, the complexity of the tumor microenvironment and host immunity requires further exploration through multicentric studies and more extensive biomarker panels.<br /><br />---<br /><br />## Conclusion<br /><br />Our comparative study indicates that combined general-epidural anesthesia is associated with a less pronounced perioperative decline in cellular immunity and improved 3-year survival outcomes in patients with non‑small cell lung cancer, compared to total intravenous anesthesia. These findings suggest that anesthetic management could be a modifiable factor in enhancing postoperative immune competence and, by extension, long-term oncologic outcomes. Further large-scale, randomized trials are warranted to validate these results and to explore the underlying biological mechanisms.<br /><br />---<br /><br />## References<br /><br /> 1. Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL, editors. *Miller’s Anesthesia*. 8th ed. Philadelphia: Elsevier Saunders; 2015.<br /> 2. Harrison’s Principles of Internal Medicine. 20th ed. New York: McGraw-Hill Education; 2018.<br /> 3. Sessler DI, et al. “Anesthesia, Surgery, and the Immune System.” *Anesthesiology*. 2000;92(5):1460–1465.<br /> 4. Cata JP, et al. “Effects of anesthetic technique on immune function and oncologic outcomes.” *Journal of Clinical Anesthesia*. 2010;22(5):386–394.<br /> 5. Kehlet H, et al. “Impact of regional anesthesia on the surgical stress response and postoperative outcomes.” *British Journal of Anaesthesia*. 2012;108(2):234–242.<br /> Muhammad Khudair Abbas <br />Al-Mustaqbal University is the first university in Iraq<br /><br />