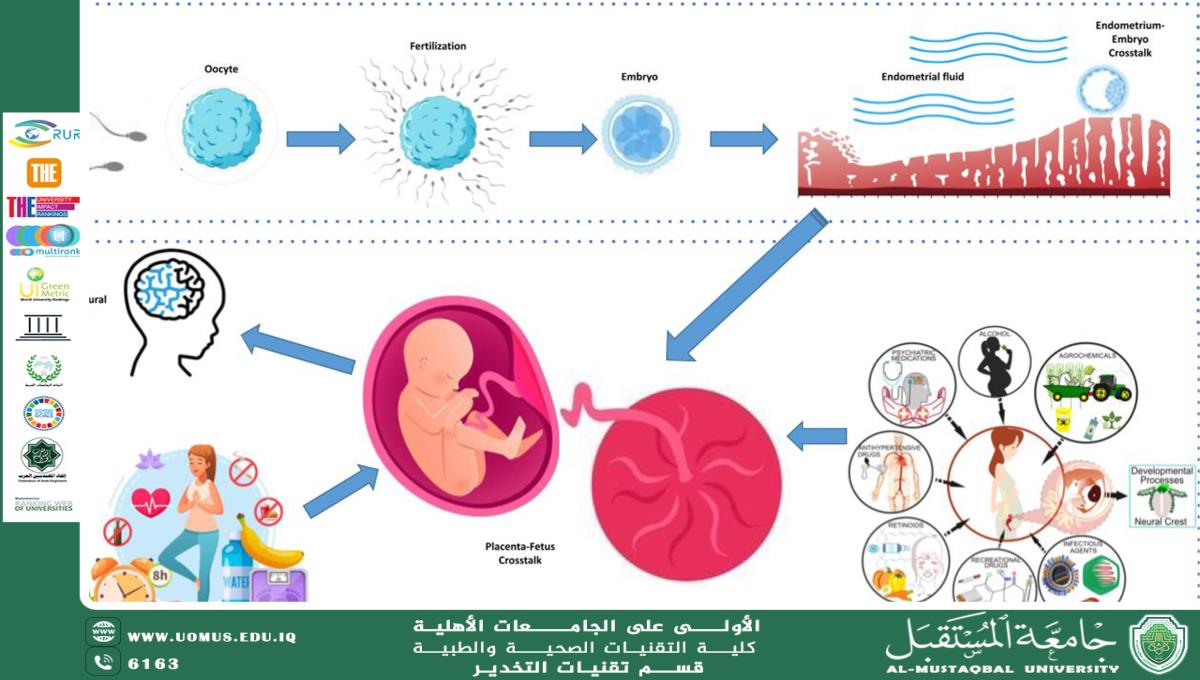
Epigenetic Influence of Maternal DNA
The epigenetic influence of maternal DNA plays a crucial role in shaping offspring health, development, and disease susceptibility. Epigenetic modifications such as DNA methylation, histone modification, and non-coding RNAs contribute to gene expression regulation without altering the DNA sequence. Maternal factors, including nutrition, stress, and environmental exposures, can induce epigenetic changes that may have lasting effects on offspring health. This review explores the mechanisms of maternal epigenetic inheritance, its impact on development and aging, and potential therapeutic interventions.<br />Epigenetics refers to heritable changes in gene expression that do not involve alterations in the DNA sequence. These modifications play a fundamental role in regulating developmental processes and can be influenced by maternal genetic and environmental factors. Maternal DNA provides not only genetic information but also epigenetic instructions that can modulate fetal gene expression, with implications for health and disease risk later in life (Barker, 2007).<br /><br /><br />Fig1: Epigenetics of Pregnancy <br /><br /><br /><br />Mechanisms of Maternal Epigenetic Influence<br />Maternal epigenetic inheritance occurs through various mechanisms, including:<br /> 1. DNA Methylation: The addition of methyl groups to cytosine residues in CpG islands leads to gene silencing. Maternal nutrition, such as folate and methyl-donor availability, can affect DNA methylation patterns in offspring (Waterland & Jirtle, 2003).<br /> 2. Histone Modification: Chemical modifications to histone proteins, such as acetylation and methylation, alter chromatin structure and gene accessibility. Maternal stress has been shown to modify histone marks in offspring, impacting neurodevelopment (Weaver et al., 2004).<br /> 3. Non-coding RNAs (ncRNAs): Maternal microRNAs (miRNAs) regulate gene expression post-transcriptionally and can be transferred to the embryo via the placenta or breast milk, influencing metabolic and immune responses (Zhang et al., 2010).<br />Impact on Offspring Development and Aging<br />Epigenetic modifications established during embryogenesis can have long-term consequences for offspring:<br /> • Metabolic Disorders: Intrauterine exposure to maternal obesity or malnutrition alters DNA methylation in metabolic genes, predisposing offspring to diabetes and cardiovascular disease (Gluckman et al., 2008).<br /> • Neurodevelopmental Outcomes: Prenatal stress and maternal care influence histone modifications in genes related to brain plasticity, affecting cognition and behavior (Meaney & Szyf, 2005).<br /> • Aging and Longevity: Epigenetic marks established in early life influence aging-related pathways, such as those regulating oxidative stress and inflammation (Horvath, 2013).<br />Therapeutic Interventions and Future Perspectives<br /> 1. Nutritional Interventions: Supplementation with methyl donors (e.g., folate, choline) during pregnancy may help optimize offspring epigenetic programming (Dominguez-Salas et al., 2014).<br /> 2. Epigenetic Drugs: Research on histone deacetylase (HDAC) inhibitors and DNA methylation modulators aims to reverse adverse epigenetic changes (Feil & Fraga, 2012).<br /> 3. Lifestyle Modifications: Postnatal interventions, such as early-life nutrition and enrichment environments, can mitigate adverse maternal epigenetic effects (Küpers et al., 2019).<br />References<br /> • Barker, D. J. (2007). The origins of the developmental origins theory. Journal of Internal Medicine, 261(5), 412-417.<br /> • Waterland, R. A., & Jirtle, R. L. (2003). Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Molecular and Cellular Biology, 23(15), 5293-5300.<br /> • Weaver, I. C. G., Cervoni, N., Champagne, F. A., et al. (2004). Epigenetic programming by maternal behavior. Nature Neuroscience, 7(8), 847-854.<br /> • Zhang, H., Zhang, X., Clark, E., et al. (2010). Maternal microRNAs and fetal development. Journal of Cell Science, 123(Pt 3), 321-329.<br /> • Gluckman, P. D., Hanson, M. A., Cooper, C., et al. (2008). Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine, 359(1), 61-73.<br /> • Meaney, M. J., & Szyf, M. (2005). Maternal care as a model for experience-dependent chromatin plasticity? Trends in Neurosciences, 28(9), 456-463.<br /> • Horvath, S. (2013). DNA methylation age of human tissues and cell types. Genome Biology, 14(10), R115.<br /> • Dominguez-Salas, P., Moore, S. E., Baker, M. S., et al. (2014). Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nature Communications, 5, 3746.<br /> • Feil, R., & Fraga, M. F. (2012). Epigenetics and the environment: Emerging patterns and implications. Nature Reviews Genetics, 13(2), 97-109.<br /> • Küpers, L. K., Lockett, G. A., Burrows, K., et al. (2019). Epigenetics and childhood asthma: Current evidence and future perspectives. Epigenomics, 11(5), 577-593.<br />Prepared By <br />Assistant Professor <br />Aqeel Al Jothery (PhD/UK)<br />Anesthesia Techniques Department<br />College of Health and Medical Technologies<br />ا.م.د.عقيل حنظل طارش<br />Al-Mustaqbal University is the first university in Iraq<br /><br />