Producing benzene from crude oil
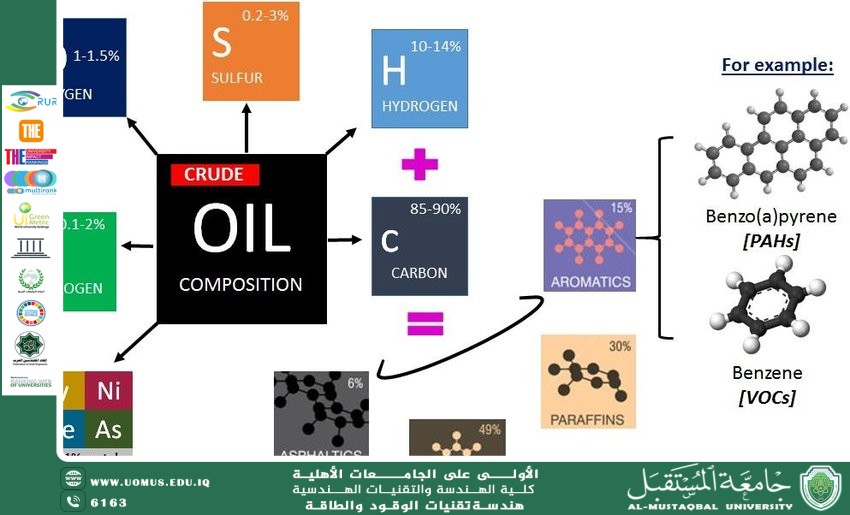
<br />Producing benzene from crude oil<br />Dr. Malik Mustafa Mohammed<br />SDG 9 Industry, Innovation and Infrastructure<br />SDG 15 Life on Land <br /><br />Producing benzene from crude oil<br />Producing benzene from crude oil involves a series of well-defined industrial processes, primarily centered around refining and chemical conversion. Here's a structured overview of the key steps:<br />1. Fractional Distillation of Crude Oil<br /> - Crude Oil Separation: Crude oil is first heated and introduced into a fractional distillation column, where it separates into fractions based on boiling points.<br /> - Naphtha Fraction: Benzene precursors are concentrated in the naphtha fraction(C5–C10 hydrocarbons, boiling range ~30–200°C). This fraction is targeted for further processing to produce petrochemicals, including aromatics.<br />Pre-Treatment: Hydrotreating<br /> - Impurity Removal: The naphtha fraction undergoes hydrotreating** (reaction with hydrogen under pressure) to remove sulfur, nitrogen, and other impurities that could poison catalysts in subsequent steps.<br />3. Catalytic Reforming<br /> - Aromatization Process: The purified naphtha is fed into a catalytic reforming unit (e.g., Platforming process). Here, under high temperature (495–525°C), pressure (20–35 bar), and a platinum-based catalyst, non-aromatic hydrocarbons (paraffins and naphthenes) undergo:<br /> -Dehydrogenation: Converting cyclohexanes (naphthenes) to aromatics (e.g., cyclohexane → benzene).<br /> - Dehydrocyclization: Converting straight-chain alkanes to aromatics (e.g., hexane → benzene).<br /> -Isomerization: Rearranging molecules to form more stable aromatic structures.<br /> - Reformate Stream: The output, called reformate, is a mixture rich in aromatics (benzene, toluene, xylenes) and hydrogen (a byproduct).<br />4. Aromatic Extraction and Purification<br /> -Solvent Extraction: Aromatics are separated from non-aromatics using solvents like sulfolane, NMP, or glycol, which selectively dissolve aromatics.<br /> - Distillation: The aromatic-rich stream is distilled to isolate individual components:<br /> - Benzene (boiling point: 80°C) is separated from toluene (111°C) and xylenes (~140°C) viafractional distillation.<br /> - Hydrogenation: Benzene may be further purified to remove trace impurities (e.g., sulfur) via hydrogenation.<br /> 5. Alternative Sources<br /> - Pyrolysis Gasoline (PyGas): A byproduct of steam cracking (used to produce ethylene/propylene), PyGas contains 40–60% aromatics. It is similarly processed via extraction and distillation to recover benzene.<br /> - Toluene Conversion: Excess toluene can be converted to benzene via:<br /> - Hydrodealkylation: Toluene + H₂ → Benzene + Methane.<br /> - Disproportionation: Toluene → Benzene + Xylenes.<br /> Key Considerations<br /> - Catalyst Sensitivity: Impurities like sulfur must be rigorously removed to prevent catalyst poisoning.<br /> - Economic Factors: Benzene production is often integrated with gasoline production (via reforming) or ethylene manufacturing (via steam cracking).<br />Summary Flowchart<br />Crude Oil → Fractional Distillation → Naphtha → Hydrotreating → Catalytic Reforming → Reformate <br />→ Solvent Extraction → Distillation → Pure Benzene <br />Additional Routes: PyGas Processing or Toluene Conversion.<br />This process is central to the petrochemical industry, supplying benzene for downstream applications in plastics, resins, and synthetic materials.<br />Al-Mustaqbal University The First University in Iraq<br /><br />