How to Produce Green Ammonia
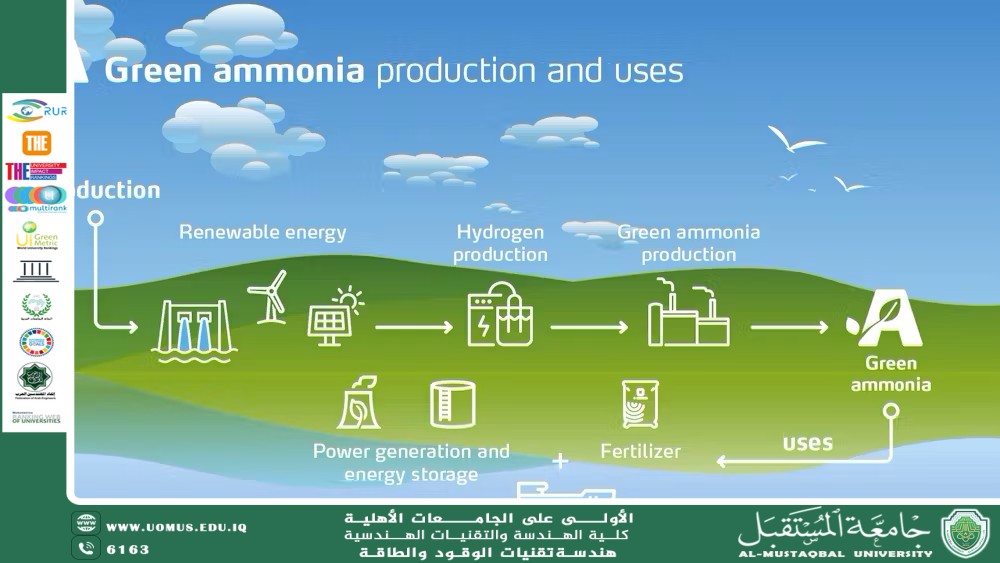
How to Produce Green Ammonia<br />Assist. Prof. Malik Mustafa Mohammed<br /><br />• SDG 7: Affordable and Clean Energy<br />• SDG 9: Industry, Innovation and Infrastructure<br />• SDG 12: Responsible Consumption and Production<br />Green ammonia is synthesized with minimal carbon emissions, primarily using renewable energy and sustainable feedstocks. Below is a detailed breakdown of the production process, based on the latest research and industry practices: <br /> 1. Hydrogen Production via Water Electrolysis <br />- Process: Split water (H₂O) into hydrogen (H₂) and oxygen (O₂) using renewable electricity (e.g., solar, wind). <br /> - Key Technologies: Proton-exchange membrane (PEM) electrolyzers or alkaline electrolysis. <br /> - Renewable Integration: Excess solar/wind energy powers the electrolysis, ensuring a carbon-free hydrogen.<br /> 2. Nitrogen Extraction from Air <br />- Process: Separate nitrogen (N₂) from atmospheric air via cryogenic distillation or pressure-swing adsorption. <br /> - Note: Nitrogen constitutes ~78% of air, making it a readily available feedstock.<br /> 3. Ammonia Synthesis via the Haber-Bosch Process <br />- Core Reaction: Combine hydrogen and nitrogen under high pressure (150–250 bar) and moderate temperatures (400–500°C) using an iron-based catalyst. <br /> - Optimization : Modern plants use advanced catalysts (e.g., ruthenium-based) to reduce energy consumption.<br /> 4. Alternative Methods for Green Ammonia Synthesis <br />While the Haber-Bosch process dominates, emerging technologies aim to improve efficiency and reduce costs: <br />- Electrochemical Synthesis: Directly produce ammonia from water and nitrogen using renewable electricity, bypassing hydrogen storage. <br /> - Example: Protonic ceramic electrochemical cells show promise for decentralized production.<br />- Photocatalytic Production: Use solar energy to drive nitrogen reduction reactions (NRR) in photocatalytic systems. <br /> - Advantage: Eliminates the need for high-pressure reactors[citation:26]. <br />- Biomass-Derived Ammonia: Gasify biomass to produce hydrogen, then apply Haber-Bosch.<br />- Solar-Driven Thermal Processes: Concentrated solar power provides heat for nitrogen separation and synthesis.<br /> 5. Storage and Distribution <br />- Storage: Ammonia is stored as a liquid at -33°C or under pressure (~10 bar at 25°C). <br />- Challenges: High energy demand for compression/liquefaction. <br /> Key Advantages of Green Ammonia <br />- Carbon-Free Fuel: Suitable for shipping, power generation, and heavy industry. <br />- Energy Density: Higher than liquid hydrogen, simplifying transport. <br />- Existing Infrastructure: Leverages decades of ammonia handling experience (e.g., fertilizer industry).<br /><br /> Challenges and Innovations <br />- Energy Intensity: Electrolysis and Haber-Bosch require significant renewable electricity input. <br />- Catalyst Development: Research focuses on low-temperature/pressure catalysts to cut costs.<br />- Scalability: Pilot plants (e.g., Siemens’ wind-powered facility) test feasibility.<br /> Alternative Methods for Green Ammonia Synthesis: Technical Breakdown <br />Below is a detailed analysis of emerging technologies for green ammonia production, based on the search results and scientific literature. These methods aim to reduce reliance on the energy-intensive Haber-Bosch process and leverage renewable energy or novel chemical pathways: <br /> 1. Electrochemical Ammonia Synthesis <br />- Process: <br /> - Uses renewable electricity to drive nitrogen reduction reactions (N₂ + 3H₂O → 2NH₃ + 1.5O₂) in electrolytic cells. <br /> - Key approaches include: <br /> - Redox-Mediated Systems: Electron transfer via redox mediators (e.g., Li/LiOH) to fix nitrogen.<br /> - Proton-Conducting Electrolytes: Use ceramic or polymer electrolytes to facilitate proton transport for nitrogen reduction[citation:18]. <br /> - Lithium Cycling : A three-step process involving LiOH electrolysis, Li nitridation, and Li₃N hydrolysis to produce NH₃.<br />- Advantages: <br /> - Operates at ambient conditions (no high pressure/temperature).<br /> - Decouples hydrogen production from synthesis, avoiding storage challenges.<br />- Challenges: <br /> - Low Faradaic efficiency (energy loss due to competing reactions like hydrogen evolution).<br /> - Catalyst durability and nitrogen activation barriers.<br /> 2. Photocatalytic Ammonia Synthesis <br />- Process <br /> - Solar energy drives nitrogen reduction using photocatalysts (e.g., TiO₂, g-C₃N₄) under ambient conditions.<br /> - Example: Visible-light-driven amination of biomass-derived substrates (e.g., α-hydroxyl acids) using NH₃[citation:28]. <br />- Advantages: <br /> - Utilizes abundant solar energy, ideal for decentralized production. <br /> - No fossil fuel-derived hydrogen required. <br />- Challenges : <br /> - Low quantum efficiency due to rapid charge recombination.<br /> - Limited nitrogen adsorption on photocatalyst surfaces. <br /> 3. Plasma-Catalytic Synthesis <br />- Process: <br /> - Non-thermal plasma generates reactive nitrogen species (e.g., N atoms, ions) that react with hydrogen on catalysts.<br /> - Combines plasma physics with catalytic surfaces to lower activation energy. <br />- Advantages: <br /> - Operates at mild temperatures/pressures compared to Haber-Bosch.<br /> - Compatible with renewable electricity (e.g., wind/solar-powered plasma reactors).<br />- Challenges: <br /> - High energy consumption for plasma generation[citation:15]. <br /> - Catalyst deactivation from plasma-induced erosion[citation:15]. <br /> 4. Biomass-Derived Ammonia <br />- Process: <br /> - Gasify biomass (e.g., agricultural waste) to produce hydrogen, then apply Haber-Bosch or electrochemical synthesis. <br /> - Alternatively, photocatalytic conversion of biomass into amino acids using NH₃ as a nitrogen source.<br />- Advantages: <br /> - Utilizes renewable carbon sources, reducing fossil dependency. <br /> - Synergizes with carbon capture if CO₂ is sequestered during gasification. <br />- Challenges: <br /> - Competition with food/feed production for biomass[citation:11]. <br /> - Energy-intensive preprocessing of biomass[citation:11]. <br /> 5. Solar-Driven Thermal Processes <br />- Process: <br /> - Concentrated solar power (CSP) provides heat for nitrogen separation from air and hydrogen production. <br /> - Example: Solar thermal reactors replace fossil-fueled Haber-Bosch heaters. <br />- Advantages: <br /> - Eliminates fossil-derived process heat. <br /> - Compatible with existing ammonia infrastructure. <br />- Challenges: <br /> - High capital costs for CSP infrastructure. <br /> - Intermittency issues without thermal energy storage. <br /><br /> 6. Hybrid Systems <br />- Process: <br /> - Combine electrochemical, photocatalytic, and thermal methods (e.g., solar-thermal-assisted electrolysis).<br /> - Example: Photoelectrochemical (PEC) water splitting produces hydrogen for Haber-Bosch.<br />- Advantages: <br /> - Balances efficiency and scalability. <br /> - Leverages multiple renewable energy inputs. <br />- Challenges: <br /> - System complexity and integration costs. <br /> Key Innovations and Future Directions <br />1. Catalyst Development: <br /> - Nitrogenase-inspired catalysts (e.g., FeMo-based) and single-atom catalysts improve N₂ activation. <br /> - Ru/K catalysts reduce Haber-Bosch energy use by 20%.<br />2. Modular Small-Scale Plants: <br /> - Alkaline electrolyze-based systems (1–5 MW) enable decentralized production.<br />3. Lifecycle Analysis: <br /> - Green ammonia must achieve <0.5 kg CO₂/kg NH₃ to qualify as carbon-free. <br /> "Al-Mustaqbal University – The No. 1 Private University in Iraq"<br /><br /><br />