Carnot Cycle Analysis and Its Role in Understanding Thermal Efficiency
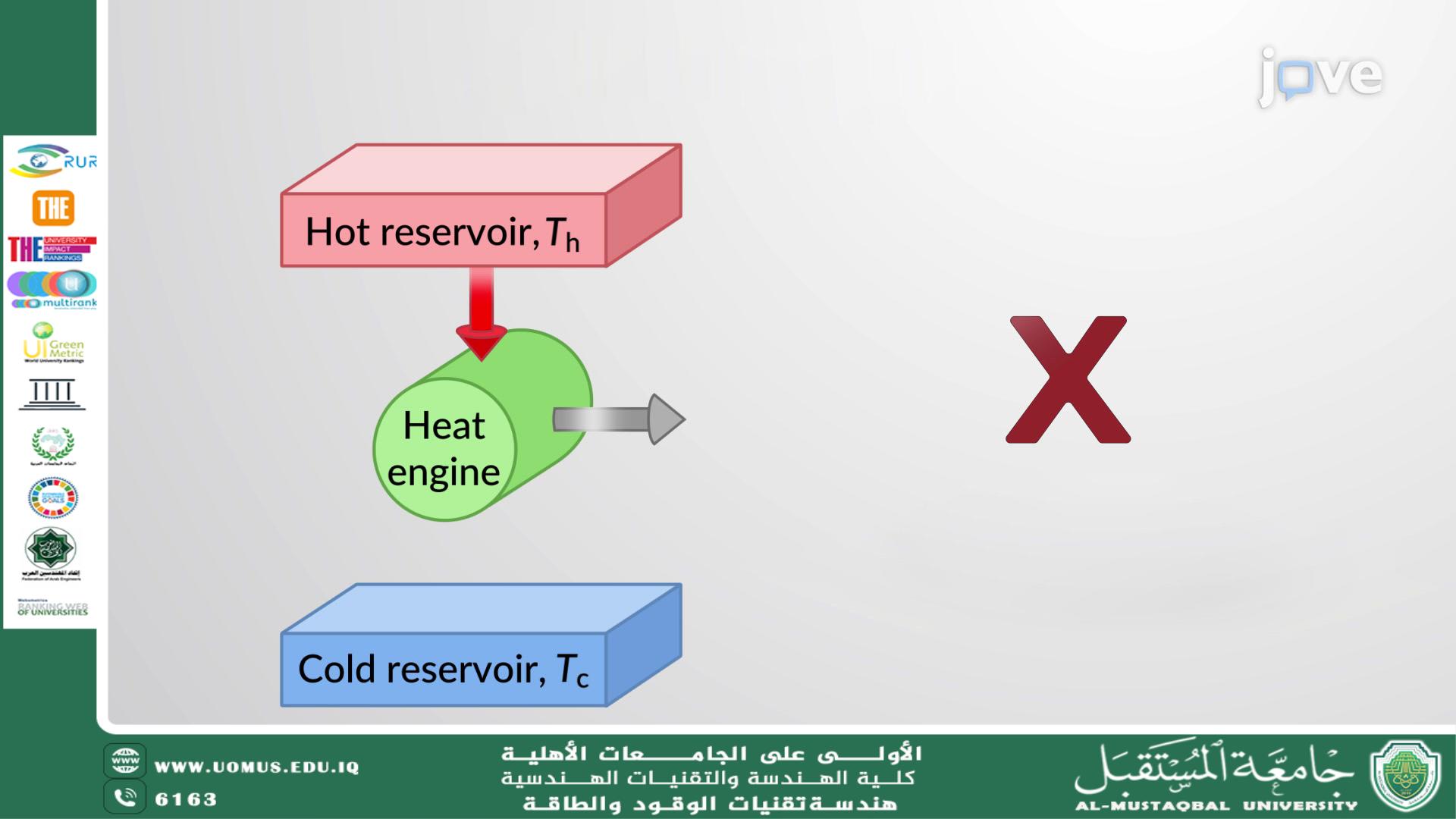
Carnot Cycle Analysis and Its Role in Understanding Thermal Efficiency<br />Eng. Nourhan Thamer Assi<br /><br />Relevant Sustainable Development Goals (SDGs)<br />Goal 7: Affordable and Clean Energy<br />Goal 9: Industry, Innovation and Infrastructure<br />Goal 12: Responsible Consumption and Production<br />Goal 13: Climate Action<br /><br />Introduction<br />The Carnot cycle is one of the most important theoretical models in thermodynamics. It helps engineers and scientists understand the fundamental limits of thermal efficiency. Though it is an idealized concept, it plays a vital role in evaluating and designing real-world energy systems. This article explores how the Carnot cycle works, its importance, and its applications in engineering fields.<br /><br />Understanding the Carnot Cycle<br />The Carnot cycle consists of four reversible steps that involve a working substance, usually an ideal gas, exchanging heat with two thermal reservoirs—one hot and one cold. These steps are:<br />Isothermal Expansion at High Temperature<br />The substance absorbs heat from the hot reservoir while expanding at a constant temperature.<br />Adiabatic Expansion<br />The substance continues to expand without any heat exchange, causing its temperature to decrease.<br />Isothermal Compression at Low Temperature<br />Heat is released to the cold reservoir while the substance is compressed at a constant lower temperature.<br />Adiabatic Compression<br />The temperature of the substance increases again as it is compressed, without exchanging heat, returning to its original state.<br />This cycle forms a closed loop, returning the system to its initial state while doing useful work.<br /><br />Importance of the Carnot Cycle<br />A Benchmark for Efficiency<br />The Carnot cycle represents the highest possible efficiency that any heat engine can achieve when operating between two temperature limits. This makes it a standard for comparing real-world systems.<br /><br />Foundation of the Second Law of Thermodynamics<br />The cycle illustrates the idea that no engine can convert all heat energy into work. Some energy will always be lost, often in the form of waste heat. This principle is key to understanding why real engines can never be 100% efficient.<br /><br />Guidance for Engineers<br />While real engines cannot fully replicate the Carnot cycle, the concept helps engineers identify how close their designs come to the ideal. It also helps highlight sources of energy loss and where improvements can be made.<br /><br />Understanding the Quality of Energy<br />The Carnot cycle shows that not all energy is equal—some forms are more useful than others. Thermal energy, for example, can be difficult to convert entirely into work, especially when the temperature difference is small.<br /><br />Applications in Engineering<br />Power Plants: The Carnot cycle helps assess and improve the performance of systems like steam and gas turbines.<br />Refrigeration and Air Conditioning: Engineers use the reverse Carnot cycle as a model to improve the efficiency of cooling systems.<br />Automotive Engineering: Thermal analysis of engines is guided by Carnot principles to reduce fuel consumption and improve energy use.<br />Aerospace Systems: High-efficiency thermal management systems are developed using concepts inspired by the Carnot cycle.<br />Sustainable Energy: The cycle supports the development of renewable and clean energy systems with better thermal performance.<br />Limitations in Practice<br />In real-life systems, perfect reversibility is impossible. Factors such as friction, heat loss, and material limitations prevent actual engines from reaching Carnot-level efficiency. However, the cycle still provides valuable insights and goals for improving performance.<br />Conclusion<br />The Carnot cycle may be theoretical, but its impact is very real. It serves as a crucial tool in the design and analysis of thermal systems across all engineering disciplines. Understanding this cycle helps improve energy efficiency, reduce environmental impact, and advance sustainable technology. As global energy demands rise, the lessons of the Carnot cycle remain essential in shaping the future of energy and engineering.<br /><br /><br />"Al-Mustaqbal University – The No. 1 Private University in Iraq"<br />